
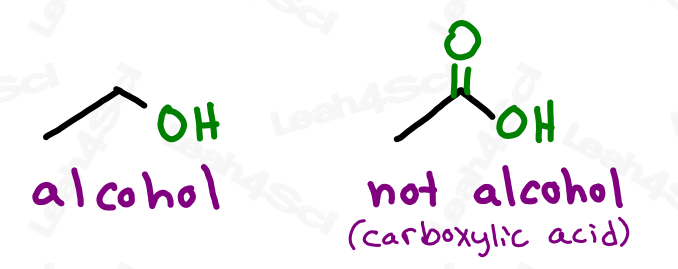
In amines the nitrogen atom alway has three bonding groups and one lone-pair. Notice in methylamine the carbon atom of the methyl group is bonded to the nitrogen. In esters the hydrogen atom bonded to the oxygen atom in the carboxylic acid is replaced with an R- group. The carboxyl group is sometime written as -COOH group in the compound.Īcetic acid, a component of vinegar is the first carboxylic acid with an R- group bonded to the carbon atom of the carboxyl group. The other oxygen atom is double bonded to the carbon.
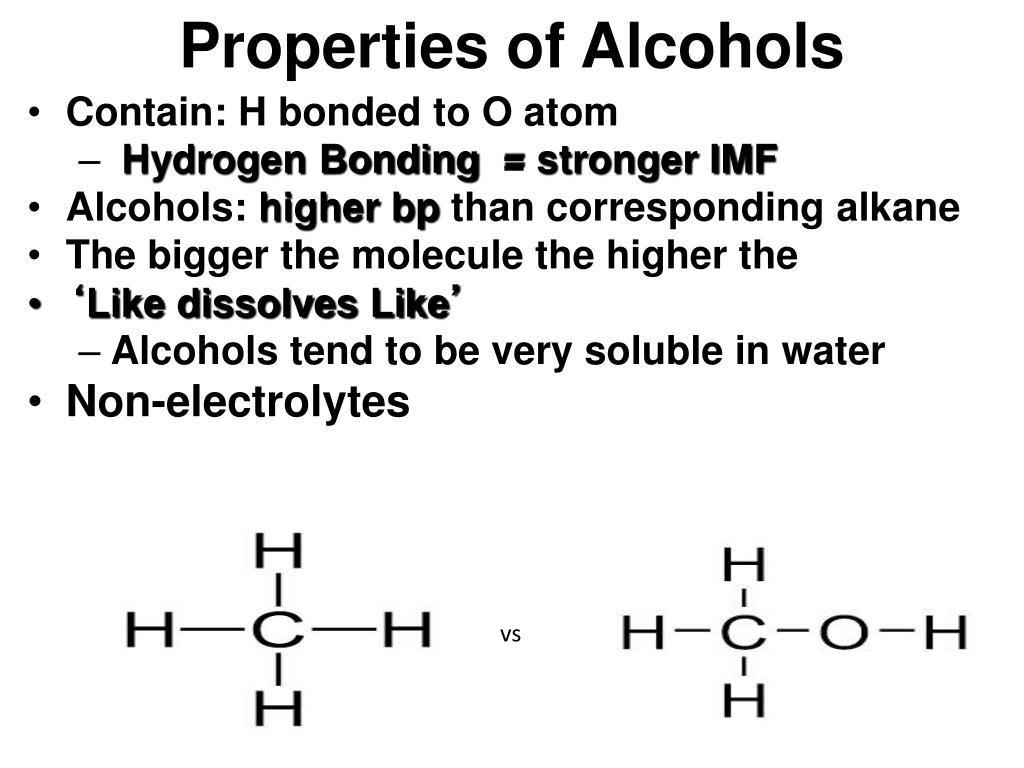
One of the oxygen atoms has a hydrogen bonded to it like in the alcohols. In carboxylic acids there are two oxygen atoms. Here the hydrocarbons are both ethyl groups. In ethers both groups attached to the oxygen atom are hydrocarbon groups. Notice in an alcohol the oxygen atom has one hydrogen atom and the other group is a hydrocarbon. Notice the condensed formula CH 3CH 2OH and the Lewis structure so you see oxygen atom is bonded to a carbon and a hydrogen.Įthers are slightly differnet compared to an alcohol. Both of these two alcohols are very important and you must be able to draw their Lewis structures and name them. Notice the condensed structural formula and the arrangement of the atoms in the Lewis structure.Įthanol is the second alcohol. The geometry is 'bent' around the oxygen atom in methanol. Methanol is similar to water, HOH, where one of the hydrogens is replaced with the methyl group. It has a hydrogen attached to it as well as the methyl group. In methyl alcohol, or methanol the red atom is an oxygen. So lets look at some examplesof each type. So I need you to recognize the functionalgroup in a Lewis structure or draw a Lewis structure containingthe functional group given it name. There are, believe it or not, other functionalgroups called aldehydes and ketones, but I'll not hold youresponsible for those.  Following is the table of the common functional groups you will encounter in organic chemistry.Functional Group Review Functional Group Review They are alcohols, ethers, carboxylic acids, esters, amines,and aromatics. The knowledge of some basic functional groups and how they react would give us tremendous leverage to tackle the problem of predicting chemical reactivity in organic chemistry. Common reactions for cholesterol and ethanol List of functional groups in organic chemistry  So all the reactions like elimination, substitution, etc would be same for these molecules as the region of reactivity is the –OH. It does not matter if ethyl alcohol has a ‘passenger strength’ of 2 C and cholesterol several, arranged in a very, very different way. The major reactions that the OH group undergoes are the common reactions of both ethyl alcohol and cholesterol.
ALCOHOL FUNCTIONAL GROUP DRIVER
Similarly if we consider the example of the ethyl alcohol and cholesterol, the driver here is the alcohol group (-OH) that both these molecules have in common. The driver at the steering is the one responsible for the whole bus’ motion hence its function, the passengers filling up the bus but don’t really do anything towards the bus.


 0 kommentar(er)
0 kommentar(er)
